

M/v (w/v) and m/m (w/w) concentrations and parts per million will be discussed in the following section (after the worked examples of percentage concentration and ppm below). To convert from ppm by mass to ppm by volume, divide by the. For example, a sample with a volume concentration of 25 µl/l will have a mass concentration of 252.65 66 mg/l.
For mineral grains (clay, silt and sand sizes), this will typically be 2.65 g/cm3. V/v concentration is NOT the same as a %(v/v) concentration To convert from ppm by volume to ppm by mass, multiply by the density of the particles.

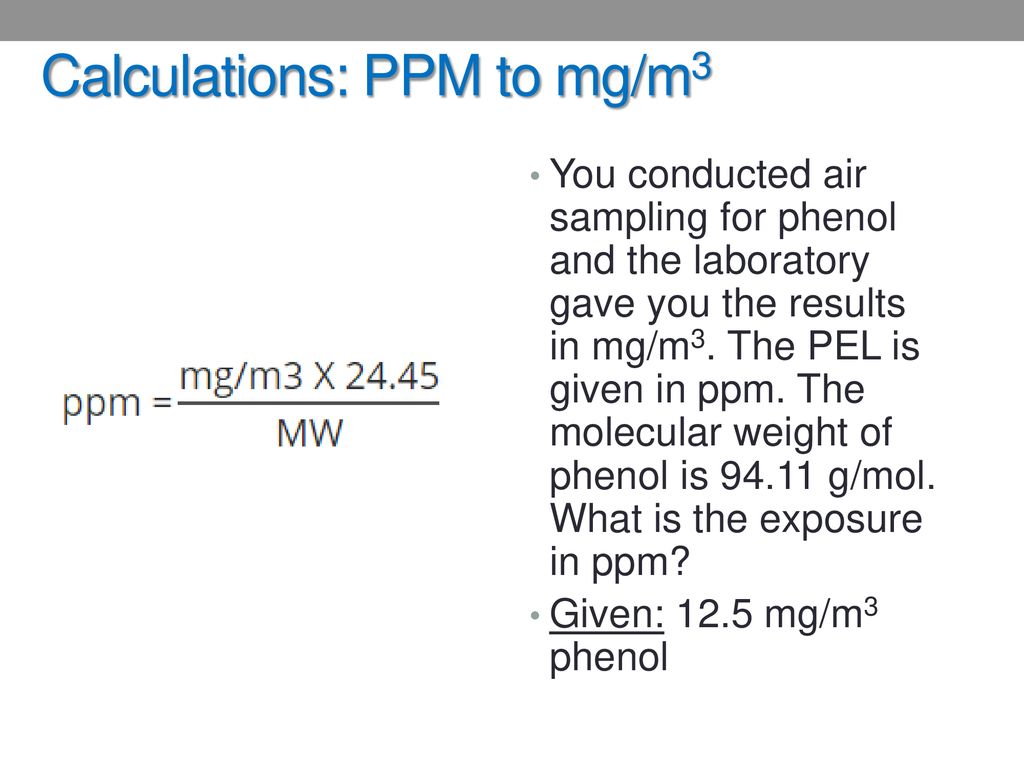
M/m concentration is NOT the same as a %(m/m) concentration W/v concentration is NOT the same as a %(w/v) concentration M/v concentration is NOT the same as a %(m/v) concentration Where x is the value of the percentage concentration This is true for other similar percentage concentrations:įor mass/volume percentage concentrations (m/v% or w/v%):įor mass/mass percentage concentrations (m/m% or w/w%): We could write a mathematical expression equating parts per hundred (a percentage) and parts per million (ppm) as shown below: you want a volume of your solute and a volume of your solution, or a mass of. The first thing you need to do is get a measurement of the amount of solute or substance you’re interested in, then get the same type of measurement for the whole solution, e.g. How would we convert that to a concentration in parts per million (ppm) ? In general, though, you can always calculate PPM using one approach and a simple formula. This means that there are 9 000 parts of NaCl in every 1 000 000 parts of the solution.īut the concentration of a solution is sometimes given as a percentage.Ī percentage concentration tells you how many parts of solute are present in 100 parts of solution.įor example, the ethanol content in wine is often given as about 12%(v/v), that is, 12% of the volume of the wine is ethanol, or, there are 12 parts of ethanol in every 100 parts of solution. Recall that, in general, concentration tells you how much solute is present in a solution.Ī concentration in parts per million (ppm) tells you how many parts of solute are present in 1 000 000 parts of solution.įor example, a saline solution is a dilute aqueous solution of sodium chloride, NaCl (aq), with a concentration of 9 000 ppm. Parts per million and Percentage Concentration Calculations


 0 kommentar(er)
0 kommentar(er)
